COVID-19 vaccine related GK Question
1 -COVID-19 vaccine is scheduled for roll-out in india - 16th January 2021
TYPE Covid-19 vaccines, under Indian Government supply
1.COVISHIELD
2. COVAXIN
Precautions se related point
Authorized Age Group - 18 years and above
Co-administration of vaccines- no, : If required, COVID-19 vaccine and other vaccines should be separated by an interval of at least 14 days
Second dose Interchangeability of COVID-19 Vaccines - not permitted (Second dose should also be of the same COVID-19 vaccine which was administered as the first dose)
Special precautions:
Vaccine should be administered with caution in persons with a history of any bleeding or coagulation disorder (e.g., clotting factor deficiency, coagulopathy or platelet disorder).
Contraindications for COVID-19 Vaccination
Persons with history of:
Anaphylactic or allergic reaction to a previous dose of COVID-19 vaccine
Immediate or delayed-onset anaphylaxis or allergic reaction to vaccines or injectable therapies, pharmaceutical products, food-items etc.
Pregnancy & Lactation:
Pregnant & Lactating women have not been part of any COVID-19 vaccine clinical trial so far. Therefore, women who are pregnant or not sure of their pregnancy; and lactating women should not receive COVID-19 vaccine at this time
Provisional / temporary contraindications: In these conditions, COVID vaccination is to be deferred for 4-8 weeks after recovery
Persons having active symptoms of SARS-CoV-2 infection.
SARS-COV-2 patients who have been given anti-SARS-CoV-2 monoclonal antibodies or convalescent plasma
Acutely unwell and hospitalized (with or without intensive care) patients due to any illness
Following conditions are not contraindicated for COVID vaccines
Persons with a past history of SARS-CoV-2 infection (sero-positivity) and or RT- PCR positive illness History of chronic diseases and morbidities (cardiac, neurological, pulmonary, metabolic, renal, malignancies) Immuno-deficiency, HIV, patients on immune-suppression due to any condition (the response to the COVID 19 vaccines may be less in these individuals)
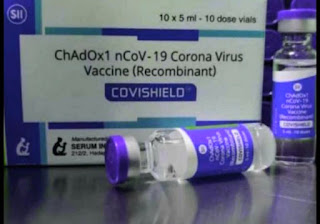
Type of Vaccine
COVISHIELD
Recombinant COVID-19 vaccine based on Viral Vector Technology
No. of doses in each vial -10
Shelf life -6 months
Route -Intramuscular (IM) Injectable
Dose -0.5 ml each dose
Course -2-doses
Schedule - 4-weeks apart
Expiry date available on vial- Yes
Vaccine Vial Monitor (VVM)- Not Available
Physical Appearance Vaccine- Clear to slightly opaque, colourless Vaccine to slightly brown
Vaccination during Pregnancy - Not recommended
Vaccination < 18 years - age Not recommended
Vaccination to Lactating- Not recommended mother
Storage and transportation - +2°C to +8°C at all levels
Not applicable Open Vial Policy
Freeze Sensitive - Yes (Discard the vaccine vial, if “frozen’ or “frozen and thawed'
found Discard the vial, if -Solution is discoloured or visible Presence of particulate observed
Some mild AEFI may occur like-injection site tenderness, injection site pain, headache, fatigue, headache myalgia, malaise, pyrexia, chills and dizziness-giddiness, tremor, sweating, cold, arthralgia, nausea
Paracetamol may be used to provide symptomatic relief from post vaccination adverse reactions
intramuscular injections, COVISHIELD should be given with caution to individuals with thrombocytopenia
2. COVAXIN
Whole-Virion Inactivated Corona Virus
No. of doses in each vial -20
Shelf life -6 months
Route -Intramuscular (IM) Injectable
Dose -0.5 ml each dose
Course -2-doses
Schedule - 4-weeks apart
Expiry date available on vial- Yes
Vaccine Vial Monitor (VVM)- Not Available
Physical Appearance Vaccine- whitish translucent
Vaccination during Pregnancy - Not recommended
Vaccination < 18 years - age Not recommended
Vaccination to Lactating- Not recommended mother
Storage and transportation - +2°C to +8°C at all levels
Not applicable Open Vial Policy
Freeze Sensitive - Yes (Discard the vaccine vial, if “frozen’ or “frozen and thawed'
found Discard the vial, - if Presence of particulate matter or coloration
Some mild AEFI may occur like - injection site pain, headache, fatigue, fever, body ache, abdominal pain, nausea and vomiting,dizziness-giddiness, tremor, sweating, cold,cough and injection site swelling
Shake well, before use
Use of Chloroquine and Corticosteroids may impair antibody response.
0 टिप्पणियाँ:
एक टिप्पणी भेजें